PACE
The PACE project is currently in non-clinical development. The first human clinical trial will focus on the “treatment of inveterate sickle cell leg ulcers”.
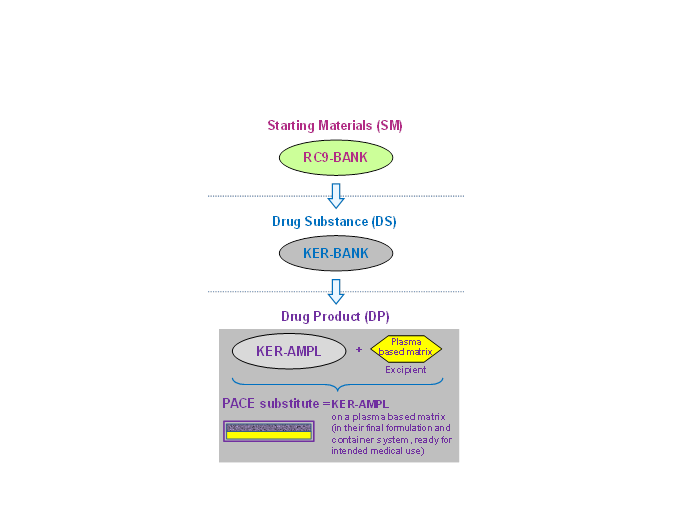
The pharmaceutical product consists of amplified keratinocytes deposited on a plasma-based matrix (excipient). The keratinocytes are derived from a hESC line: the RC-9.
The RC-9s were amplified and banked to form a bank of frozen cells: the RC9-BANK, which is a starting material (MD). The RC-9s are then differentiated into keratinocytes, which are amplified to form a frozen cell bank: the KER-BANK, which is the drug substance.
The first human clinical trial (PASS-ES) is planned for early 2025: “Safety, tolerability, and efficacy of a human embryonic stem cell-derived epidermal substitute for the treatment of inveterate sickle cell leg ulcers: A first-in-man, multicenter, phase I/II trial.”
The trial will be conducted in four dermatological centers in the Paris region (Ile de France) that treat most sickle cell leg ulcers. These four centers are representative of the sickle cell population because half of the births of sickle cell patients occur in the Paris area.
An additional center is being evaluated in the department of Guadeloupe, as this site has the largest number of patients with sickle cell leg ulcers in the French Caribbean.
Last Update :
- Keratocyte development batch: ready
- Ongoing non-clinical studies
- The first batch of clinical IMPs should be produced in Q4 2024
- Clinical trial application expected in Q3 2024
- Recruitment of the first patient is planned for Q1 2025